Chemical contortionists: the chemistry of Shape Memory Alloys

Over time, a wide variety of metals have played crucial roles in the construction of buildings and other types of infrastructure. With the constant development of modern day technology and science, the emergence of different kinds of metals such as alloys are ever-evolving, aided by the use of more advanced science to understand and analyse the fine (micro) structures of these alloys being used.
A brief overview of SMAs
Shape Memory Alloys are a class of alloys that are known for their ability to return to a predetermined shape, that after deforming can do so through heating, allowing for the crystalline structure to revert to its original shape under specific temperatures.
Well known chemists Chang and Read played crucial roles in its discovery, noting that the Martsenic transformation occurs without the diffusion of atoms (a modification in the crystal structure during a change in phase).
Other major breakthroughs included the discovery of Nitinol (the nickel-titanium alloy), which showed impressive recovery properties which lead to the recognition of the Shape Memory Alloys, and with the help of the addition of elements including Copper and Iron, could alter the temperatures in which these shape changes would occur as previously mentioned.
There are many applications of these Shape Memory Alloys, over various industries including medicine such as braces in orthodontics and electronic components.
Pseudo-elasticity (or superelasticity)
What is pseudo-elasticity and why is it relevant? Pseudo-elasticity refers to the response created to relatively high stress (the magnitude of force that causes the deformation) caused by a phase transformation between the austenitic and martensitic phases of a crystal, more to come on these terms in this article!
Pseudo-elastic behaviour occurs when stress causes deformation of these alloys, then tension as the material (alloy in our example) is subjected to external forces, which then reforms to its original shape when exposed to higher temperatures. This process includes deforming the alloy at high temperatures, and the material reverting to its original shape when pressure is no longer applied.
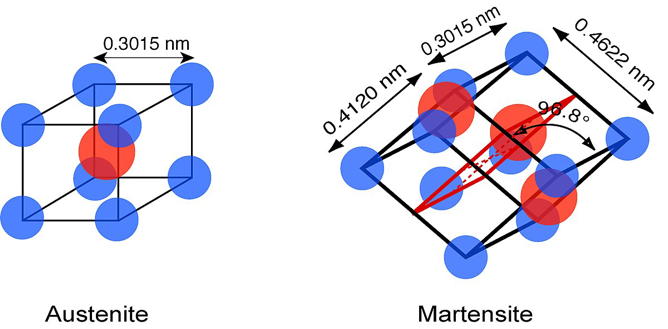
This starts with the Austenite phase, where stress causes a transformation into martensite phase.
To compare these two phases we can imagine metals as if they were made of up many building blocks, which are the atoms. Martensite and austenite phases are the two different ways in which these arrangement of ‘building blocks’ occur and arrange themselves when the metal is cooled down or heated.
The austenite phase is a structural phase in which metals are heated. At high temperatures, these atoms gain mobility, rearranging them into an ‘organised face-centred cubic’ (FCC) arrangement.
The martensite phase forms when metals are cooled quickly from high temperatures, preventing atoms from settling into ordered arrangements. Instead, they are cooled in a distorted state creating a ‘distorted body-centred tetragonal’ (BCT) structure.
As the level of stress increases, inelastic strains (deformation in a material that is not fully reversible once stress is removed) are developed until the transformation is over. Once the transformation is completed, the increase in the strains are able to be seen. Therefore, to allow us to see the true wonders of SMA’s, the reverse process must take place. This includes removing the external forces being applied, returning to the Austenite stage, and reverting back to its shape. This transformation here, is what exhibits the shape memory effects, which results in the superelastic behaviour of the SMA.
The uses of SMAs
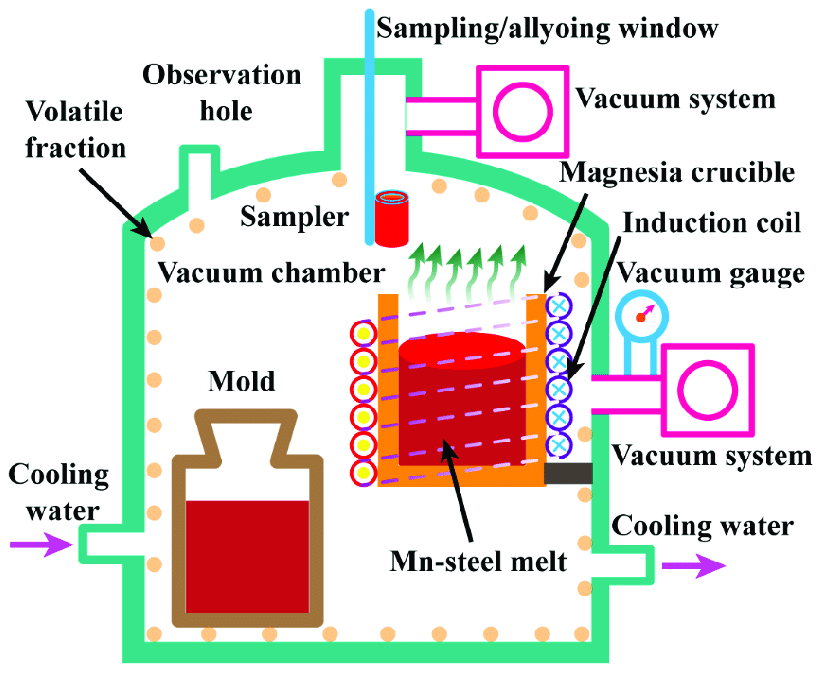
As seen previously, SMAs have a wide range of application, and each of these types of alloys produced come in different forms to accommodate this. Examples of this discussed previously include the well known SMA Nitinol, or the use these chemical powders for further synthesis to occur. From a practical perspective, this can include casting and other well known metallurgy methods, and the process of making these alloys can involve several other techniques. A better known method is Vacuum Induction Melting, which is used to produce SMA’s on large scales for commercial use. A picture of this process can be seen below:
Overall, shape memory alloys can be used in our everyday technology and science thanks to their unique properties and shape recovery characteristics, and with the ever-evolving science of our modern day and age, it is likely that its ability for further application will expand.