Makeup to Die For: Pale and Deadly

Painted Illusions
The Renaissance was a world of spectacle of lavish fabrics, gilded palaces and faces that gleamed like porcelain. A world where beauty was no longer just a marker of status, but an art form, a living canvas where power and seduction were painted in layers of white, red and gold. Yet, under the delicate brushstrokes and shimmering powders , the cosmetics of the time were chemical cocktails of lead, mercury and nightshade. Here, we are going to to peel back the alabaster masks of the Renaissance to examine the science that made – and unmade – these living works of art.
The Façade of Frost
In the parlours of Renaissance courts, pale skin extended beyond being more than just a preference to being propaganda; an unblemished, moonlit complexion spoke of nobility, leisure and divine favour. The secret? Venetian ceruse (also known as “Venetian white” or “blanc de céruse de Venise”), a lead-based, luxurious white paste applied in thick, luminous layers across the face, neck and décolletage. It promised a porcelain glow, while in reality, it invited a cascade of biochemical disaster.
Ceruse was created through a grotesque marriage of elegance and decay: thin sheets of metallic lead were immersed in pots of vinegar, also known as acetic or ethanoic acid (CH3COOH) then allowing it to sit for weeks, sealed tightly in dung heaps. However disgusting, the warm, moist environment accelerated the corrosion, with the lead reacting with the acid and the carbon dioxide from the decomposition to form a flaky, white crust of basic lead carbonate (2PbCO₃·Pb(OH)₂). This was then scraped off, ground into a powder and mixed with rosewater or various oils and fats to form a smooth, white foundation.

At first, ceruse worked like a dream; it smoother over blemishes, blurred pores and created an almost celestial glow. However, skin is not an inert environment and with repeated use, the compound broke down in the slightly acidic environment of human sweat, rich in lactic acid, sebum and carbon dioxide, subtly destabilising the lead carbonate into more soluble lead salts, notable lead acetate (Pb(CH₃COO)₂). This compound easily penetrated the epidermis, entering the dermal blood vessels and, from there, circulating through the whole body. Inside the body, these Pb²⁺ ions mimicked Ca²⁺ and Zn²⁺, binding to enzyme active sites as well as ion channels and structural proteins. By replacing zinc in enzymes such as δ-aminolevulinic acid dehydratase (ALAD) and ferrochelatase, lead disrupted the biosynthesis of haem, crucial for oxygen transport and mitochondrial respiration, leading to hypochromic anaemia (ironically, the pallor from this anaemia often matched the painted complexion) and fatigue, just as the Egyptian kohl had done. Likewise, this biometal mimicry also had neurological consequences with the Pb²⁺ interfering with voltage-gates calcium channels (VGCCs) in neurones, derailing synaptic transmission and disrupting mood, cognition, memory and movement.
Over time, the reactive oxygen species (ROS), such as hydroxyl radicals and superoxide anions caused by the Pb²⁺ ions targeted lipid bilayers in skin cells, particularly the unsaturated fatty acids in cell membranes, undermining the skin’s structural integrity, thus accelerating the very signs of aging the ceruse was meant to mask. Mitochondrial dysfunction followed this as the electron transport chain faltered under this oxidative pressure, leading to ATP depletion, cellular senescence and apoptosis. This meant long-time users of ceruse found their skin erupting with sores and cracking at the seams and, in an attempt to hide the damage, applied more of the very poison that caused it in an endless loop.
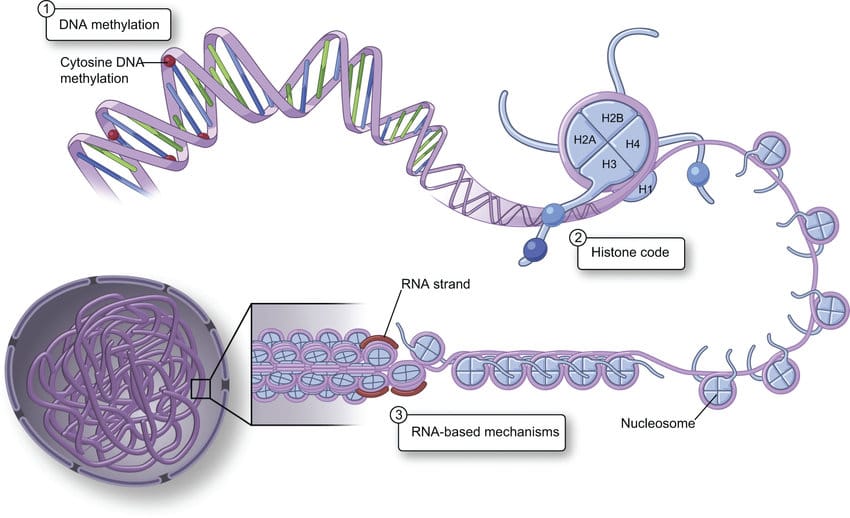
Recent research also shows that lead doesn’t just merely poison the body, it may actually reprogram it. Chronic exposure to Pb²⁺ ions induces widespread disruption of epigenetic regulation, the mechanism by which gene expression is altered without changing the underlying DNA sequence itself. The lead interferes with the activity of DNA methyltransferases (DNMTs), resulting in global hypomethylation which, by a widespread reduction in DNA methylation across an entire genome, destabilises genomic integrity and can activate oncogenes due to an increase in mutation rates (the genes responsible for cancer). Simultaneously, it promotes hypermethylation at promoter regions of tumor suppressor genes which silences their protective function. It also disrupts histone modification enzymes, including histone deacetylases (HDACs) and histone acetyltransferases (HATs). By altering the acetylation pattern on lysine residues of histone tails, lead exposure tightens or loosens chromatin structure inappropriately, misregulating transcriptional access to key genes involved in cell proliferation, apoptosis, immune signalling and neurodevelopment. Unfortunately, these epigenetic effects have proven not to be transient, with some persisting across cellular generations. Furthermore, there is emerging evidence, particularly from transgenerational animal studies, that maternal lead exposure can actually effect the epigenome of their offspring, leading to neurodevelopmental deficits, altered stress responses and an increases susceptibility to diseases. Thus, the legacy of ceruse was not confined to the women who wore it and the toxic imprint of beauty may have echoed into the lives of their children.
Due to repeated application, the lead bioaccumulated in the bones, where it disrupted hormonal signalling, especially in the hypothalamic-pituitary-gonadal axis contributing to irregular menstruation, miscarriage and infertility, quietly reinforcing ideals of delicacy and fragility. Unfortunately, these dangers did not disappear with something as simple as a washcloth as lead has a biological half-life of over 20 years meaning that even if a women were to stop using ceruse, her skeleton would slowly leach Pb²⁺ ions back into the bloodstream during times of illness, menopause or pregnancy. In some cases, modern osteoarcheological studies using ICP-MS (inductively coupled plasma mass spectometry) have revealed lead levels in 16th century remains hundreds of times above the modern safety thresholds. Today, the antidote for this is known: chelation therapy, using agents like EDTA (ethylenediaminetetraacetic acid) or dimercaprol to bind to Pb²⁺ and facilitate its excretion by binding to heavy metals (such as the Pb²⁺) thus preventing these species from binding to other areas in the body and causing harm.
The Belladona Gaze
In Renaissance Italy, beauty was in the eye, and not just of the beholder. A wide, glassy and dilated pupil was considered the height of sensuality, a symbol of eternal infatuation and mysterious allure. Women of the court turned to extracts of Atropa belladonna, a deadly nightshade, which artificially enlarged their pupils creating a gaze of perceived desire and vulnerability, while the name itself, belladonna, or “beautiful woman” hinted at its cosmetic use.
The active compound here was atropine, a tropane alkaloid, a class of alkaloids characterised by a tropane ring which have a bicyclic structure made up of six-membered and five-membered rings connected at a single carbon atom (another more well known example of a tropane alkaloid is cocaine, derived from the coca plant). This acts as a potent anticholinergic which block the action of acetylcholine (ACh), a neurotransmitter in the central and peripheral nervous systems. Due its structure as a tertiary amine (the nitrogen is binded to three carbon atoms) with a bicyclic ring system, atropine is relatively hydrophobic, therefore rendering it lipophilic enough to cross biological membranes, including the blood-brain barrier. When dropped into the eyes, atropine competitively binds to muscarinic acetylcholine receptors, specifically the M3 subtype found in the smooth muscle of the iris sphincter. The M3 muscarinic acetylcholine receptor (M3R) is a G protein-coupled receptor (GPCR), a large family of cell surface receptors that play a crucial role in signalling between cells, and is involved in various physiological processes such as smooth muscle contraction, glandular secretion and inflammation. Normally acetylcholine (ACh) stimulates these receptors to constrict the pupil in response to light, but atropine, by blocking these receptors, induces mydriasis (prolonged pupil dilation) regardless of the lighting.
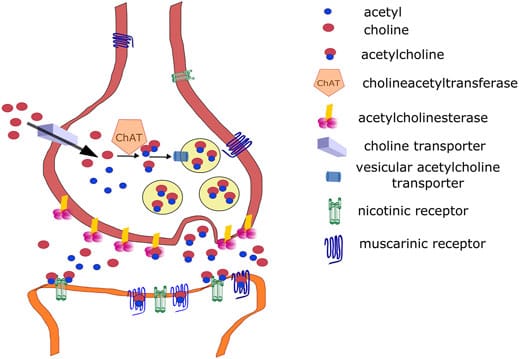
At first the effects were merely aesthetic, with an intensified, velvety gaze. But as with all things alchemical, such attempts at beauty extracted a price. Vision blurred die to the paralysis of ciliary muscles in the eyes responsible for accommodation. Near objects became indistinct, creating a dreamy, vacant stare, a side effect often misread as demureness. The eye’s natural photoprotection was lost, exposing the retina to damaging levels of ultraviolet light and accelerating oxidative stress in retinal cells, thus increasing the risk of macular degeneration, an eye condition that causes blurred vision or reduced central vision due to the breakdown of a part of the retina called the macula responsible for central vision.
Due to systemic absorption through the conjunctiva and the nasolacrimal duct (the small tube that drains tears from the eye to the nasal cavity), atropine’s action was not confined to the eye. Instead it entered the bloodstream, unleashing widespread inhibition of the parasympathetic nervous system (PNS), a division of the autonomic nervous system, which is primarily responsible for the effects of the body’s “rest and digest” functions which acts against our “fight or flight” response facilitated by the sympathetic nervous system and helps maintain a relaxed state by regulating processes like heart rate, digestion and breathing. This had various effects on the body: salivation dryed up, gastrointestinal motility slowed and heart rate climbed, all signs of anticholinergic syndrome. The central nervous system, especially rich in muscarinic receptors, was particularly vulnerable causing users to report hallucinations, disorientation and vivid, nightmarish dreams, all manifestations of the atropine binding to M1 muscarinic acetylcholine receptors (M1R) in the hippocampus and cerebral cortex which contributed to neuronal excitability and synaptic plasticity as well as other cognitive functions (the loss of such M1Rs has also been linked to cognitive decline and memory impairment in Alzheimer’s disease).
Mechanistically, this blockade of the M1 receptors disrupted the finely tuned cholinergic signalling responsible for memory formation, attention and spatial navigation, while chronically this could mimic early-onset cognitive decline. In modern toxicology, atropine is recognised as a deliriant, capable of inducing anticholinergic toxidrome, a clinical syndrome of “hot as a hare, blind as a bat, dry as a bone, red as a beet, mad as a hatter, full as a flask,” or more simply put, symptoms which may include things like hot, dry skin, flushed appearance, mydriasis, tachycardia (elevated heat rate), decreased bowel sounds and urinary retention. Pharmacogenomic studies suggests that individuals with polymorphisms, when there are two or more variant forms of a specific DNA sequence, in cytochrome P450 enzymes, a superfamily of enzymes primarily found in the liver responsible for metabolising drugs and other substances in the body, especially CYP2D6 and CYP3A4 which are crucial for atropine metabolism, may have had prolonged or exaggerated responses. In these instances, even very low doses could result in profound systemic effects, further complicating the long term safety of this beauty regime. Likewise, in rare, yet very possible, vases, excessive atropine exposure could sway the autonomic balance towards life threatening arrhythmias (irregular heartbeats) or even seizures. Despite all of this, none of it deterred its popularity; after all wide pupils meant widened prospects of status, admiration, marriage, no matter the cost to the vision, mind or our very cells.

Polished to death
To be flawless was to be divine. In the courts of Europe and the Qing dynasty palaces alike, smooth, pale skin whispered of purity, prestige and power, but when beauty is measured in clarity, every blemish becomes a threat. And, just like the Romans years before them, so came the powders, the ointments and the mercury, a promise of porcelain perfection. Women applied creams laced with mercury (II) chloride (HgCl2) or rubbed mercury amalgams (mercury alloys) into their skin, believing in the metal’s mystical ability to erase sunspots, freckles and the uneven tints of time; and in a way, it worked… until the mirror cracked.
Once absorbed through the skin, particularly easily in areas where the stratum corneum, the outermost layer of the epidermis is the thin, or damaged by repeated exfoliation, the Hg2+ ions seek out thiol (-SH) groups on cysteine residues of proteins. These sulfhydryl groups are essential to maintaining the tertiary 3D structure of many enzymes (which provide the specific shape for their active sites), particularly those involved in cellular defence, such as glutathione reductase and thioredoxin reductase. By irreversibly binding to these sites, mercury denatures the enzymes, crippling their antioxidant capacity and leaving the cells to oxidative attack, caused by an imbalance between the body’s production of free radicals and its ability to neutralise the with antioxidants, leading to potential cellular and tissue damage. For example, glutathione, the body’s most abundant antioxidant, becomes oxidised, thus opening the door to a cascade of reactive oxygen species.
Within the mitochondria, mercury interferes with complexes I and III of the electron transport chain (ETC), responsible for facilitating electron transfer and pumping protons to generate a proton gradient used for ATP synthesis, thus blocking the synthesis of ATP and leading to a buildup of superoxide radicals. Without energy, cells falter. With ROS, they die, either by apoptosis (controlled cell death) or necrosis (uncontrolled cell death). Unfortunately, the skin, that ever-visible canvas, reflects the damage first: inflammation, erythema, peeling, the very afflictions the mercury was meant to cure. And like a snake eating its own tail, the treatment demanded more treatment.
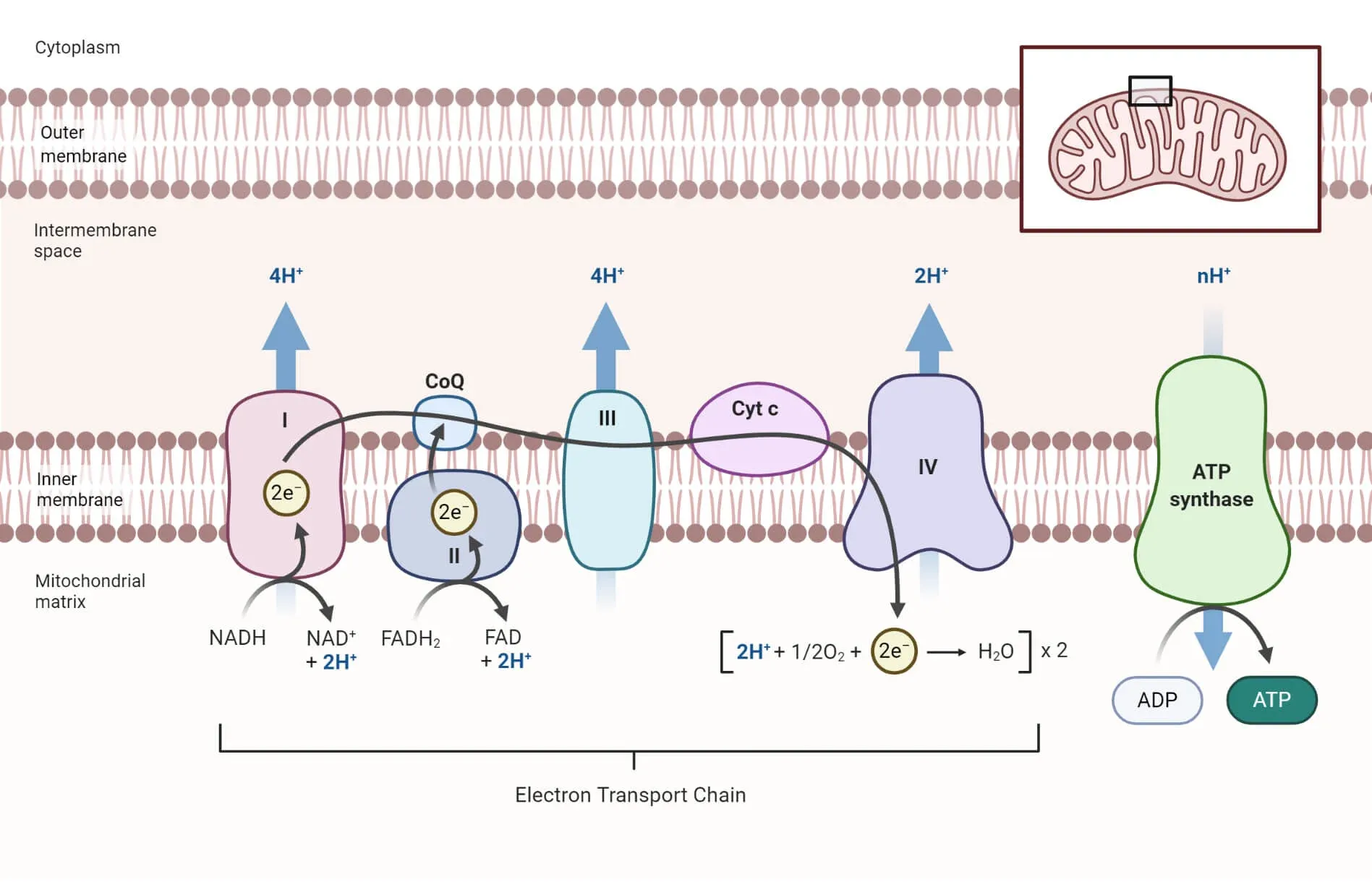
However, its effects did not end at the skin. Mercury, especially in its divalent form, is poorly excreted, thus accumulated in the kidneys, the primary route of elimination. Here, it binds to proteins in the proximal tubules, leading to tubular necrosis and glomerular dysfunction. Chronic exposure leads to nephrotic syndrome: proteinuria (where excess proteins are present in the urine), oedema (swelling caused by build up of fluid in tissues) and eventual renal failure, sometimes misattributed at its time to vague “female maladies” or melancholia, a term used since the time of the ancient Greeks to describe a feeling of intense sadness and hopelessness. Furthermore, mercury, like lead, can venture across the blood-brain barrier by mimicking essential cations or going along cysteine transporters as mercury-cysteine conjugates, thus infiltrating the central nervous system. In the brain, Hg²⁺ disrupts neurotransmitter synthesis, especially catecholamines, a group of neurotransmitters produced by the adrenal glands and nerve tissues in response to stress, such as dopamine and norepinephrine. Axonal transport falters and myeline sheaths deteriorate meaning synaptic integrity collapses, the result being the gradual unravelling of the self: tremors, irritability, insomnia, memory loss and, eventually, hallucinations and psychosis. Similarly, “Mad as a hatter” was no mere phrase, with hat makers too using mercury nitrate to felt wool and succumbed to the same spectral madness.
Ironically, the pursuit of physical purity tainted the psyche. The polished, unblemished face belied the decay behind it: the kidney scarring, the mitochondria flickering into silence, the mind fraying at its edges. However, in a similar process to lead, mercury doesn’t just simply poison the body, it rewrites it. Studies suggest that mercury interferes with selenoprotein (proteins containing selenocysteine (Sec), a selenium containing amino acid) function and may induce epigenetic dysregulation via histone methylation changes and oxidative DNA damage, disrupting transcriptional patterns essential to cell survival and neurodevelopment. In pregnant women, mercury traversed the placenta with ease, entering the foetal brain and impairing neuronal migration, essential for the proper functioning of the nervous system, differentiation and synaptogenesis, forming synapses between neurones: a legacy of perfection paid in another life.

In love with a lie
The pursuit of beauty in the Renaissance was a double-edged sword: refined on one side, poisoned on the other. Behind the ivory skin, widened eyes and flawless complexion lay a horrifying story of biochemistry. These women were alchemists in their own right, wielding jars of lead, belladonna and mercury in rituals of transformations into relics of an era where beauty meant sacrifice. Unfortunately, women didn’t learn their lesson here, but continued even till the 19th and 20th century where beauty turned luminous, and lethal, with a kiss of radium, a touch of arsenic and another wash of mercury.