The Chemistry of Ice Cream: Achieving the Perfect Scoop with Molecular Precision
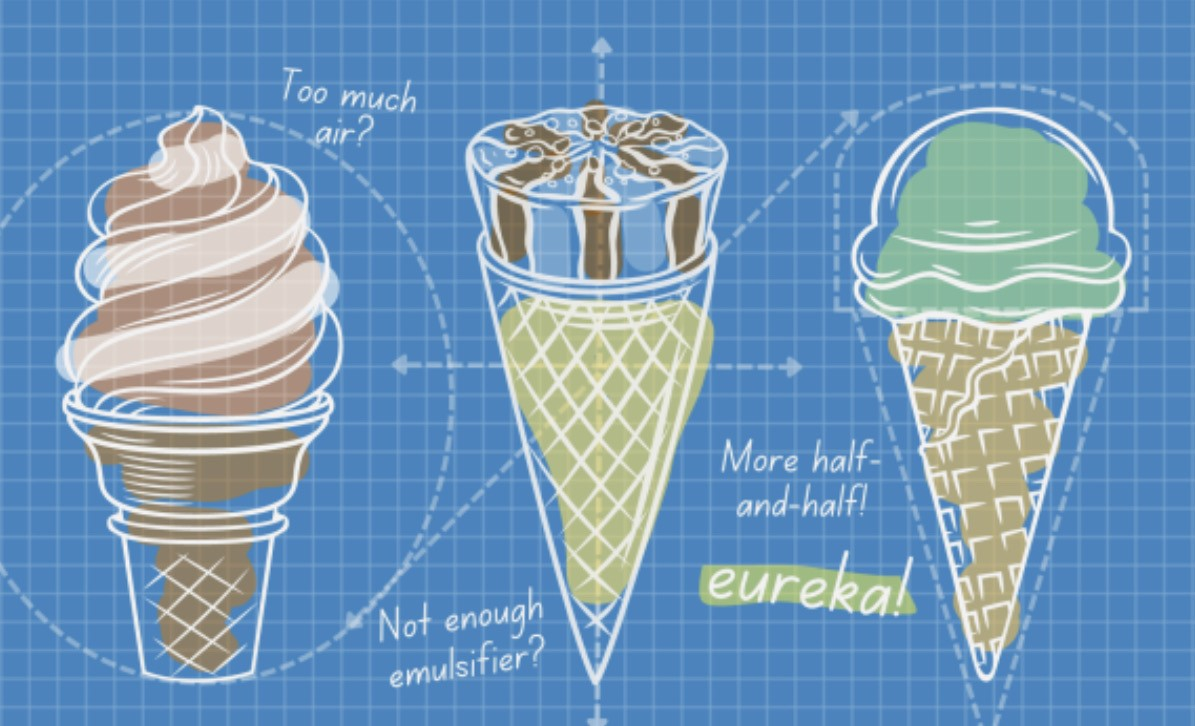
Ice cream: the sweet treat that we all love. Behind this sweet treat lies a fascinating world that bridges the gap between molecular chemistry and ice cream. There are four main components that go into the making of this treat:
Emulsifiers
The fat used in the making of ice cream, consists mainly of triglycerides (molecules consisting of glycerol and three fatty acids, hence ‘tri’ as seen by the three different colours in the diagram below) of which its chemical composition is very important, as fat content is used to determine the precise melting point of the ice cream . This is because we don’t want our ice cream to melt straight away, but we also want it to be soft enough to eat. The same goes for ice creams that use vegan ingredients such as those without dairy, and have replacement oils such as coconut oil which possess similar melting points to that of either cream or dairy, allowing for them to be suitable substitutes.
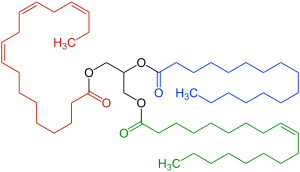
Speaking more on the fatty acids, the acyl chains (as seen below in the black diagram) found within the structure of the triacylglyercol (TAG) dictates the melting point and how the ice cream melts which is a key component in the sensation we associate when eating a soft ice cream.
To achieve that creamy taste of ice cream, we can say thanks to the emulsion transformation within ice cream between hydrophilic and lipophilic domains. The proteins within the milk act as amphiphilic (a chemical compound possessing hydrophilic and lipophilic characteristics) agents by coating the fat droplets present and therefore preventing coalescence (the merging of two elements), and the emulsifiers then further enhance the stability of our ice cream, which as a result prevents separation and enhances that creaminess. The hydrophilic molecules and hydrophobic molecules play an important role in the stability of the emulsifiers too! As the emulsion creates a creamier texture by dispersing fat droplets into the water, facilitating milk protein coatings and therefore prevents larger molecules from forming as well as the mixing of the water.
Finally, to summarise this concept, there are two components in the emulsifier. These are the water-soluble components and the fat-soluble components (hydrophilic and lipophilic components) which allow milk proteins to combine and produce a thin membrane which surrounds droplets preventing them from forming a cluster when air is added to the ice cream being made.
Air
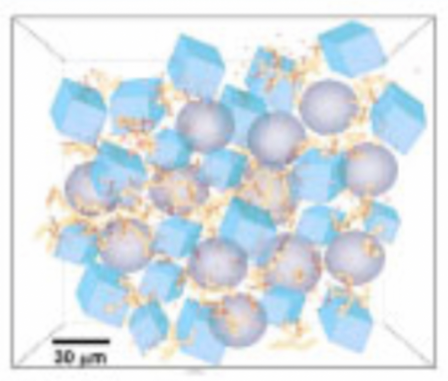
The structure of ice cream also plays a key role in what we have deemed as the perfect scoop. When ice cream is being made, it is simultaneously frozen and air is constantly added. In fact, the majority of ice creams contain a significant portion of air, and it is this proportion of air that is incorporated which differentiates the structure, ie. The factor which determines if one makes gelato or normal ice cream. The structure can be seen in the photo below:
Ice cream also spans across different types especially when travelling around the world. Such an example is sorbet. As mentioned, we need those fats and proteins in order to incorporate more air to our batch of ice cream, but with an ice cream type such as sorbet, the lack of dairy means that there is insufficient proteins and fats to allow for more air giving the sorbet its well known icy texture. This is because the greater the volume of air present within the ice cream, the faster it is able to melt. The majority of commercial ice creams have large volumes of air incorporated to allow for mass production on a large scale, and also to let the ice cream fill larger containers despite using a smaller quantity of products!
Finally, regarding texture, the commercial manufacturing of ice cream involves some more interesting chemistry! Seen below is a commercial ice cream making machine, in which a large barrel surrounded by liquid ammonia are kept below minus 30 degrees Celsius, a necessary temperature for our cold treat. The constant application of these freezing conditions and and scraping allow small ice crystals to form and become evenly distributed throughout the ice cream, where the smaller the ice crystal the smoother texture, typically found in higher quality ice creams, and a desired characteristic in making that perfect scoop. Seen below is the structure of ammonia and some ice crystals:


Flavourings, colourings and stabilisers
However, as the famous saying goes, we eat first with our eyes, and when it comes to something like ice cream, its important to consider the actual flavourings and colours, as well as stabilisers to achieve that perfect scoop. Ice cream nowadays has a huge variety of different flavours which range from all natural to man-made, as well as flavour enhancements which not only affect the taste of ice cream but its texture too. Talking more on flavouring, a study in the US showed that 59% of Americans (taken from a sample of 1000 people) preferred vanilla ice cream, so lets talk a bit more on this popular flavour. Despite a few of the components of ice cream being natural, proper flavour can be achieved from artificial flavourings, and in the case of vanilla is the flavouring compound Vanillin which is added to the ice cream during production. The right image below is the skeletal formula of Vanillin:
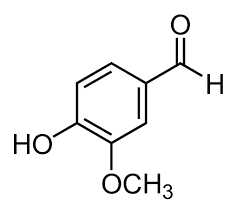
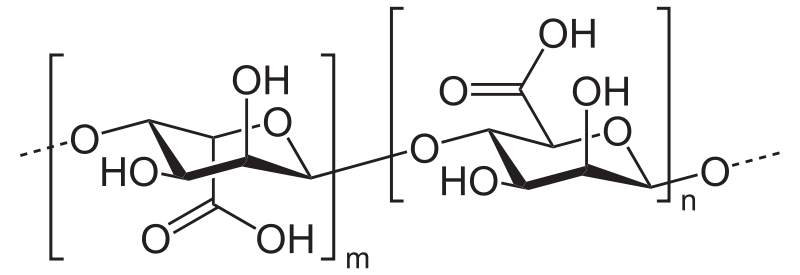
Stabilisers are used in the commercial production of ice cream where it is mass produced, and prevent the breaking down of the ice cream which gives it a longer shelf life, beneficial from an economic perspective by improving the profit margin due to an increased efficiency through reduced waste. These can usually be found from plant-based materials including seaweed and fungi. And, by combining all of these components, it gives us a great idea as to what ice cream actually is. A commonly used stabiliser is Alginic acid seen below, derived from an algae.
Sweetners
The final component to discuss is sweeteners. We wouldn’t really like ice cream that much if it wasn’t sweet, so its really important to ice cream makers to ensure that their treat is indeed sweet to make sure its consumption is as pleasant as possible. Typically, sugars and syrups are use d to add sweetness as well as aiding the texture of the ice cream. Not only does it help with the taste, but it also lowers the freezing point of the ice cream mixture preventing the ice cream from becoming rock-solid, an undesirable characteristic especially when it comes to achieving the perfect scoop.

Conclusion
To conclude this brief article, lets have a quick look at the manufacturing process of ice cream. Base ingredients including water, sugar milk and fat are used, where more fat and less air provides a lovely dense texture. Pasteurisation is next where the ice cream base is heated to kill any harmful bacteria (we don’t want that!!), and homogenisation. This breaks up the fat droplets mentioned earlier in this article, allowing for them to be dispersed evenly further enhancing the texture and stability of the ice cream, typically conducted at high pressures. Ageing is next where the base is allowed to settle to improve that texture (very important for that perfect scoop, hence mentioned a lot in this article). This usually lasts throughout a 24 hour period. Then freezing where a commercial freezer is used to freeze the base also incorporating air and flavourings (both which have been discussed). Liquid nitrogen can also be added sometimes, its not vital but is used by some manufacturers to get that super smooth ice cream, perfect for scooping, sometimes found in dedicated ice cream parlours. Finally, hardening. The ice cream is further stabilised in the freezer until is stable enough to be scooped, and interestingly, at home some people find that after opening their ice cream and putting back, upon scooping ice cream a second time it is much harder, and this is due to the difference in settings of the two freezers, and the freezing method is what lies within the most crucial step in achieving the perfect scoop.
To close this article, some information on freezing purely due to its significance in achieving the ideal scoop. Before freezers were invented, ice cream was made using natural ice and snow mixed with a variety of ingredients, but after the discovery of whats called the “freezing point-depression’ it revolutionised ice cream making allowing for the liquid base to freeze to a solid, again giving us that perfect set up for a scoop. Explained in more detail, it is the combination of ice and salt to lower the freezing point of the ice cream in order for other liquids to also be frozen, and is still used in many commercial ice cream machines today!
